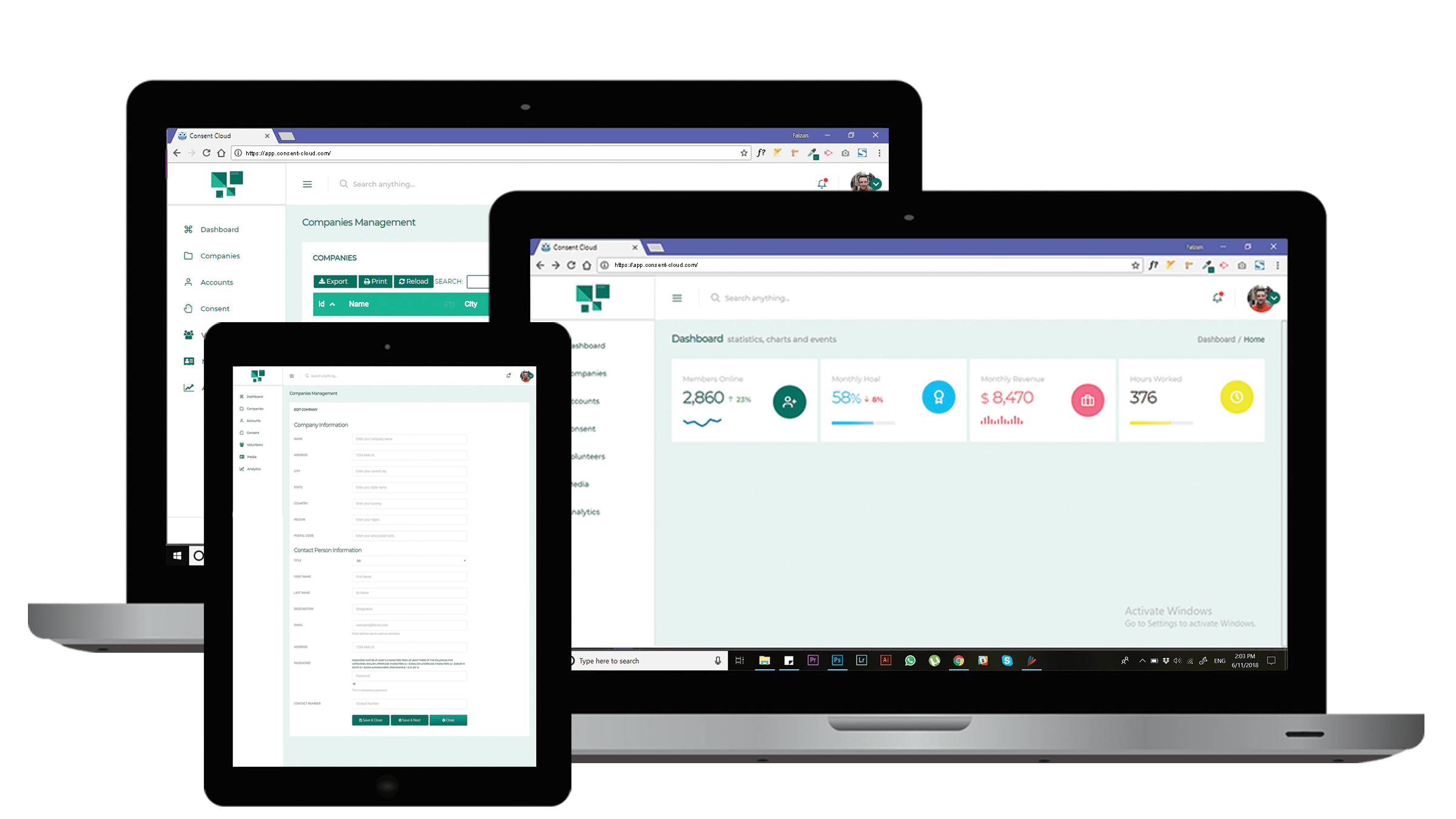

Improved version control
Increased transparency
Improved protection of patients through enhanced insight
Continuous improvement of consent content
Improved efficiencies
Ability to focus on patient's targeted questions and area of concern
Timely Insight into consent related activities
Improved ongoing oversight
Versioning control, less quality risk
Less administrative burden
The presentation of the consent with multi media offers more features than paper consents are link to additional resources for supporting an informed decision. The electronic informed consent helps in better understanding the clinical research study. The participants can start the consenting process at home and in some cases not have to go to a research site, if feasible for particular study.
Patients in clinical trials are also closely monitored by experts who are specialized in their specific condition. Taking part in a clinical trial is different from having regular care from your own doctor always have the support of a team of health care providers, who will likely monitor health closely. If an investigational treatment turns out to be effective, trial participants will have benefited from the therapy long before it’s available to patients everywhere.
The user can obtain the information he needs to know about the data remotely. A remote data platform allow users to configure alerts that are triggered in real-time, as soon as certain events occur. These alerts can be configured to automatically send an email or text message, so the notified users do not need to wait until they log in to a computer to know about the event. Remote data platforms help users review data more easily is by automatically highlighting important information.
The consent is designed to explain the clinical trial process clearly using terminology that is easy to understand by a layperson. Provide patients clear, easy-to-understand clinical trial information and reduce complex and time-consuming explanations. Improve review/approval process, patients recruitment process and reduce dropout rates.
Participants can start the consent and take a break as needed, and start where they left off. Participants may feel less pressure to sign straight away. They have time without feeling anxious and can involve friends and family in their decisions.